FIP makes it a priority to publish the latest developments in pharmacy practice, pharmaceutical sciences, related global news and prominent events. Our multi-media approach to keeping members, partners and peers informed of what is and will be affecting their area of practice or science is key to keeping information flowing through the FIP network.

Promoting and communicating the value of self-care: Self-care does not mean no care
Watch video•Contact information

Pharmaceutical supply chain resilience in humanitarian crises
Watch video•Contact information

Advancements in Inhalable Nano-Drug Delivery Systems for Targeted Lung Therapy
Watch video•Contact information

Empoderar a los farmacéuticos para apoyar la deshabituación tabáquica en la región de las Américas
Watch video•Contact information

Improving collaborative care: Strengthening inter- and intra-professional relationships
Watch video•Contact information

Pharmacists’ role in advancing self-care and universal health coverage
Watch video•Contact information

Empowering pharmacists to support tobacco cessation in Europe
Watch video•Contact information

PPR SIG 2024 Online
Watch video•Contact information

Pharmacy practice research summer meeting for PhD students, postdoctoral fellows and supervisors
Watch video•Contact information

Empowering pharmacists to support tobacco cessation in Africa
Watch video•Contact information

Empowering pharmacists to support tobacco cessation in the Eastern Mediterranean Region
Watch video•Contact information

Management of common skin conditions and ailments in the community pharmacy
Watch video•Contact information

Pharmacists' role in supporting health behaviour change
Watch video•Contact information

Empowering pharmacists to support tobacco cessation in Southeast Asia
Watch video•Contact information

Empowering pharmacists to support tobacco cessation in Western Pacific Region
Watch video•Contact information

Automation in Hospital Pharmacy – Implementation and Insights
Watch video•Contact information

Importance of Global Harmonization for Patient Access to Generic Drugs
Watch video•Contact information

World Environment Day: Advancing pharmacy for a more sustainable world
Watch video•Contact information

Leveraging pharmacists for tobacco cessation: New policy tools, evidence, and success stories
Watch video•Contact information

Reducing Inequities in the Health and Pharmacy Workforce: The Importance of Maternity and Parental Leave Policies
Watch video•Contact information

Enabling pharmaceutical education transformation in the Western Pacific region: Launch of FIP-UNESCO UNITWIN Centre for Excellence
Watch video•Contact information

Closing the medicines supply gap in the African region: The East African perspective
Watch video•Contact information

“Learnings from Policy Leaders in Pharmacy around the World: Eastern Mediterranean Region/Middle East
Watch video•Contact information
Using data to shape vaccination strategies – Launch of FIP’s global intelligence report on the role of pharmacists in vaccination
Watch video•Contact information

Vaccination services in pharmacy: what’s the price to pay?
Watch video•Contact information

What can the Curriculo PFP Programme for Pharmaceutical Scientists do to enhance my professional development and enable me to effectively manage my career?
Watch video•Contact information

Safe disposal of medicines: A helpful solution for our planet
Watch video•Contact information

E-labelling & Digital Transformation in Pharmacy
Watch video•Contact information

Cultural and Linguistic Considerations in the Development of Collaborative Services in Pharmacy
Watch video•Contact information
Pharmacy engagement in tuberculosis prevention and care
Watch video•Contact information

The role of pharmacists in the prevention and management of periodontal disease and other common oral health conditions
Watch video•Contact information

Gender Equity in the Health Workforce: Accelerating progress
Watch video•Contact information

Empowering Pharmacy Technicians: Navigating Resilience in Healthcare
Watch video•Contact information

Obesity: understanding the disease, nutritional approaches and new pharmacological interventions
Watch video•Contact information

Road Map for Drug Product Development and Manufacturing of Biologics
Watch video•Contact information

Hospital Pharmacy Practice & Achievements in EMRO. What next?
Watch video•Contact information

Welcome to Pharmabridge!
Watch video•Contact information

Mastering Writing Productivity: Tips and Tricks from Pharmacy Faculty
Watch video•Contact information

Ethics of Sustainable Healthcare
Watch video•Contact information

Optimising cancer care: community pharmacy strategies to support patients on oral treatment
Watch video•Contact information

Iron Deficiency Anaemia: Managing Symptoms and Supporting Self-Care Handbook Launch
Watch video•Contact information

Climate change and vector-borne neglected tropical diseases: Positioning pharmacy practice to drive awareness, prevention and management
Watch video•Contact information

Advancing future pharmacy and pharmaceutical sciences: Launch of the FIP AIM academic capacity assessment tool
Watch video•Contact information

Role of the Pharmacist in the Management of Heart Failure
Watch video•Contact information

Pharmacovigilance- The Changing Global Paradigm
Watch video•Contact information

Never Waste a Crisis: Disruptive Technologies in Africa during and post COVID-19 Pandemic
Watch video•Contact information

FIP Basel Statements – 2024 and beyond
Watch video•Contact information

FIP ECPG Strategic Plan 2023-2030 Launch: Setting the Goals Ahead for the Early Career Pharmacists, Pharmaceutical Scientists and Educators Globally
Watch video•Contact information

Partnerships and Opportunities with the FIP Foundation
Watch video•Contact information

Learnings from Policy leaders in Pharmacy around the World
Watch video•Contact information

Pharmacists support to medication management for people living with HIV
Watch video•Contact information

“Real-time release for dissolution testing of an oral solid dosage form – case study from industry (part I) and regulatory expectations (part II)”
Watch video•Contact information

FDA Office of Generic Drugs and Advancing Generic Drug Development
Watch video•Contact information

Sharing perspectives on the pharmacists’ needs in humanitarian settings
Watch video•Contact information

New roles of pharmacists in antimicrobial resistance: fungal disease management
Watch video•Contact information

Antimicrobial resistance and stewardship education: Supporting development of the pharmaceutical workforce
Watch video•Contact information

Integrating point-of-care testing in community pharmacy practice: Findings from a global intelligence report
Watch video•Contact information

Men's health from a pharmacy angle: Supporting men with erectile dysfunction and improving awareness of prostate cancer
Watch video•Contact information
The role of the pharmacist in Chronic obstructive pulmonary disease (COPD)
Watch video•Contact information

Reaching at-risk and vulnerable groups: Pharmacy’s role in inclusive vaccination
Watch video•Contact information

Preparing for winter: Pharmacy-based vaccination strategies (Episode 2)
Watch video•Contact information

Regulators Advisory Group: Launch of the Regulatory Self-Assessment Tool for Substandard or Falsified Medical Products
Watch video•Contact information
Particle -size Measurement and Its Impact on Drug Bioavailability
Watch video•Contact information
Unlocking Vaccine Confidence: A Pharmacy Perspective (Episode 1)
Watch video•Contact information

Celebrating the 5th Anniversary of Astana Declaration: Pharmacists contributing to primary health care
Watch video•Contact information

Vaccination: Benefits beyond specific disease prevention - Part 4
Watch video•Contact information

Enabling pharmaceutical education transformation in South East Asia: Launch of FIP-UNESCO UNITWIN Centre for Excellence
Watch video•Contact information

Pain management and counselling: how can pharmacists support patients in achieving better quality of life
Watch video•Contact information

11th FIP Global pharmacy technician symposium 2023 - Day 2
Watch video•Contact information

11th FIP Global pharmacy technician symposium 2023 - Day 1
Watch video•Contact information
Pharmacy-based Common Ailment Schemes: Findings from a FIP global intelligence report
Watch video•Contact information

Cardiovascular health - medication management and adherence
Watch video•Contact information

Learnings from Policy leaders in Pharmacy around the World
Watch video•Contact information

Mental health - Screening, referral and support by pharmacists
Watch video•Contact information

Understanding migraine and medication overuse headache: How can pharmacists better support patients?
Watch video•Contact information

Non-communicable diseases: Effective medication management and adherence
Watch video•Contact information

Vaccination: Benefits beyond specific disease prevention - Part 3
Watch video•Contact information

Designing pharmacies towards an accessible, patient-centred and service-oriented model of practice
Watch video•Contact information
Interconnection between the acute pain management and mental wellbeing
Watch video•Contact information

Experiences from the field: Re-inventing the roles of Pharmacists in Africa Post-COVID-19
Watch video•Contact information

In-Vitro Release Testing of Semisolid Topical Dosage Forms
Watch video•Contact information
Impact of regulatory reliance on expanding global access to essential medicines
Watch video•Contact information

Vaccination: Benefits beyond specific disease prevention - Part 2
Watch video•Contact information

Empowering early career pharmacists: Self-care interventions in common gastrointestinal and respiratory symptoms for enhanced patient care
Watch video•Contact information

Vaccination: Benefits beyond specific disease prevention - Part 1
Watch video•Contact information

Enabling life-course immunisation through pharmacy-based vaccination: Launch of a new FIP Pandemic Preparedness report
Watch video•Contact information

Sports pharmacy practice and education
Watch video•Contact information
Enabling life-course immunisation through pharmacy-based vaccination: Launch of a new FIP policy toolkit
Watch video•Contact information

Empowering Pharmacy Teams: Leveraging Global Insights for Self-Care Enhancement
Watch video•Contact information

Advancing Pharmaceutical Supply Chain Management: Strategies for Optimising Global Health Outcomes
Watch video•Contact information

New Approaches for Therapeutic Pulmonary RNA Delivery
Watch video•Contact information
Pharmacy technician roles and responsibilities beyond the pandemic
Watch video•Contact information

Learn, Grow, and Develop- Part 2: A Curriculo Preview with ECPG
Watch video•Contact information

Strategies and activities in the development of patient care competencies in mental health for pharmacy students and practitioners
Watch video•Contact information

Tips, Tricks, Technology, and Transitions of Care for Tiny Tots
Watch video•Contact information

The role of pharmacists in chronic kidney disease
Watch video•Contact information

A Capstone on Mentorship: Programmatic Possibilities
Watch video•Contact information

Learn, Grow, and Develop: Opportunities for Personal and Professional Growth with ECPG
Watch video•Contact information
The role of pharmacists in tobacco cessation
Watch video•Contact information

Health and self-care literacy for the management of minor ailments in the pharmacy-Reflux management, Heart burn and indigestion
Watch video•Contact information

The launch of the GbCF Handbook
Watch video•Contact information

Digital health in pharmacy: FIP online course for educators and practitioners
Watch video•Contact information

A global view of pharmacists’ needs in humanitarian settings
Watch video•Contact information

Health and self-care literacy for the management of minor ailments in the pharmacy-Sore throat, cough, cold and flu management
Watch video•Contact information

A Culture for Sustained Mentoring: Organizational Perspective
Watch video•Contact information

Health and self-care literacy for the management of minor ailments in the pharmacy-Children's fever management
Watch video•Contact information

Developing a high-quality research proposal - The FIP Foundation and ECPG Grant for Professional Innovation
Watch video•Contact information

Motivating Language: Special Considerations for Mentoring Among Diverse Groups of Colleagues
Watch video•Contact information

The role of pharmacists in the prevention and management of stress
Watch video•Contact information

Health and self-care literacy for the management of minor ailments in the pharmacy - Body pain management
Watch video•Contact information

Harmonization and Global Efforts for Safe and Effective Generic Drugs
Watch video•Contact information

The role of pharmacists in the prevention and management of sleep disorders
Watch video•Contact information
From Regional to Global: The FIP Latin America Biowaiver Project
Watch video•Contact information

Vaccines & special-risk groups: Pharmacy teams and all healthcare workers
Watch video•Contact information

Health and self-care literacy for the management of minor ailments in the pharmacy: Women’s intimate wellness and health
Watch video•Contact information

Mentoring a Brighter Future for Pharmacy
Watch video•Contact information

Supporting the Pharmacy Technician Workforce Across the Globe
Watch video•Contact information

How digital health is changing care delivery for pharmacists and improving public health
Watch video•Contact information

Reflecting on successes and looking to the future: Showcase of the FIP Women in Science and Education Programme
Watch video•Contact information

The role of pharmacy professionals in point-of-care testing
Watch video•Contact information

Precision Medicine: What will it Cost?
Watch video•Contact information
Enabling life-course immunisation through pharmacy-based vaccination: Access to data and vaccination records
Watch video•Contact information

Impact of pharmacy education on global health care system
Watch video•Contact information

The role of pharmacist in closing the gender pain gap
Watch video•Contact information

Reflux management in the community
Watch video•Contact information

Strategies to improve enrolment in schools of pharmacy - Launch of the FIP AIM Handbook
Watch video•Contact information

Enabling life-course immunisation through pharmacy-based vaccination: Regulations and prescribing
Watch video•Contact information

Pharmacists’ contributions to telehealth and telepharmacy services
Watch video•Contact information

Biorelevant Dissolution Testing
Watch video•Contact information

FIP-UNITWIN Global Summit on pharmaceutical education
Watch video•Contact information

Sports pharmacy: Global opportunities for pharmacists in the sports setting
Watch video•Contact information

Vaccines & special-risk groups: roundtable summit
Watch video•Contact information

Vaccines & special-risk groups: roundtable summit (French translation)
Watch video•Contact information

Enabling life-course immunisation through pharmacy-based vaccination: Service remuneration models
Watch video•Contact information

Accelerating Pharmaceutical Science: innovating and collaborating to fight COVID-19 and future pandemics
Watch video•Contact information

The role of pharmacists in infectious & tropical diseases prevention and management
Watch video•Contact information

The role of pharmacists in infectious & tropical diseases prevention and management (French translation)
Watch video•Contact information

Pharmacists’ contributions to HIV prevention and management
Watch video•Contact information

Long COVID and pharmacy: Managing the long-term effects of COVID-19
Watch video•Contact information

Supporting pharmacy leaders and educators for implementing competency-based education in pharmacy and pharmaceutical sciences education: Launch of the FIP CBE implementation handbook
Watch video•Contact information

Time to Take (AMS) Action! What will you do?
Watch video•Contact information

Commemorating the World Antimicrobial Awareness Week: Pharmacy taking action
Watch video•Contact information

Commemorating the World Antimicrobial Awareness Week: Pharmacy taking action
Watch video•Contact information

AMS: Tools in Action
Watch video•Contact information

Facilitar el acceso a los medicamentos: Estudios de casos de reglamentación de bioexenciones de América Latina
Watch video•Contact information
The patient voice in generic medicine development
Watch video•Contact information

Vaccines & special-risk groups: Diabetes
Watch video•Contact information

Antimicrobial stewardship: Communities in action
Watch video•Contact information

Understanding the global pharmacy supply chain for hospital pharmacies
Watch video•Contact information

Antimicrobial stewardship: Data in action
Watch video•Contact information

FIP quality assurance in pharmacy education survey
Watch video•Contact information
Pharmacy-based nutrition care: How nutrition impacts heart health
Watch video•Contact information
Launch of the FIP global competency framework for educators and trainers in pharmacy
Watch video•Contact information

Antimicrobial stewardship: Collaboration in action
Watch video•Contact information

Special event on pandemic preparedness Pharmacy’s global responses to the COVID-19 pandemic
Watch video•Contact information

Transforming pharmacy practice for improved care and management of cardiovascular diseases
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (Western Pacific Region)
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (Eastern Mediterranean Region)
Watch video•Contact information

Facilitating access to medicines: Biowaiver regulatory case studies from Latin America
Watch video•Contact information

Facilitar el acceso a los medicamentos: Estudios de casos de reglamentación de bioexenciones de América Latina
Watch video•Contact information

FIP Global Pharmacy Technician Symposium 2022 DAY 2
Watch video•Contact information

Antimicrobial stewardship: Pharmacists in action
Watch video•Contact information

FIP Global Pharmacy Technician Symposium 2022 DAY 1
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (Southeast Asian Region)
Watch video•Contact information

Global symposium: Why flu vaccination matters
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (African Region)
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (European Region)
Watch video•Contact information

Creating development goal indicators – bridging data and outcomes (Americas Region)
Watch video•Contact information

RNA-based medicines for unmet medical needs
Watch video•Contact information

Precision Medicine: Where is the technology heading?
Watch video•Contact information

Transforming pharmacy practice for improved care and management of cancer
Watch video•Contact information

Vaccines & special-risk groups: Older adults
Watch video•Contact information

Nutrition for immunity: Stronger immune systems through healthy nutrition
Watch video•Contact information

Transforming pharmacy practice for improved care and management of chronic respiratory diseases
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the Western Pacific region
Watch video•Contact information

Achieve equitable, inclusive and quality pharmaceutical education for all: Launch of the FIP toolkit for addressing inequities in pharmaceutical education
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the Americas region
Watch video•Contact information

Climate change as a risk to respiratory health
Watch video•Contact information
Co-administration of flu and COVID vaccines: Improving convenience and vaccination coverage
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the South East Asian region
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the European region
Watch video•Contact information

The role of pharmacists in preventing influenza and increasing vaccination coverage rate
Watch video•Contact information

Le rôle des pharmaciens dans la prévention de la grippe et l'augmentation de la couverture vaccinale
Watch video•Contact information

YPG Career Development Toolkit Workshop
Watch video•Contact information

Metodología GA-RxODE en ensayos clínicos de Bioequivalencia Fundamentos y aplicaciones.
Watch video•Contact information

Bioequivalencia - Estudios de Permeabilidad: principios, metodologías y análisis de datos
Watch video•Contact information

Building data and intelligence capabilities to provide evidence of impact for the profession
Watch video•Contact information

Vaccines & special-risk groups: Cardiovascular diseases
Watch video•Contact information

Vaccines & special-risk groups: Cardiovascular diseases (French translation)
Watch video•Contact information

Gut wellness and its role in immunity
Watch video•Contact information

Gut wellness and its role in immunity (Portuguese translation)
Watch video•Contact information

Gut wellness and its role in immunity (Spanish translation)
Watch video•Contact information

Delivering person-centred support for COVID-19: current and future pharmacy practice
Watch video•Contact information
The role of pharmacists in the management of irritable bowel syndrome
Watch video•Contact information

Bioequivalencia - Estudios de Disolución de Formas Farmacéuticas
Watch video•Contact information
Falsified Medicine and Misinformation on the Internet: Public Risk and Solutions
Watch video•Contact information

Indoor air pollution and health: causes, management and self-care approaches
Watch video•Contact information

Developing Global Pharmacist Travel Health Services
Watch video•Contact information

Transforming pharmacy practice for improved care and management of mental health illnesses
Watch video•Contact information

The WHO Universal Health Coverage Competency Framework: Building more holistic and integrated health systems.
Watch video•Contact information

What you need to know about healthy nutrition for pharmacy
Watch video•Contact information

Vaccines & special-risk groups: Chronic Respiratory Conditions (French translation)
Watch video•Contact information

Vaccines & special-risk groups: Chronic Respiratory Conditions
Watch video•Contact information

Bioequivalencia - Estudios de Solubilidad de Fármacos
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the Eastern Mediterranean region
Watch video• Website•Contact information

The role of pharmacists in diabetes prevention, screening, and management
Watch video•Contact information

The role of pharmacists in diabetes prevention, screening, and management (French translation)
Watch video•Contact information

Expedited Regulatory Pathways – possible in developing countries with strong reliance practices and post-authorization surveillance systems
Watch video•Contact information

Expedited Regulatory Pathways – possible in developing countries with strong reliance practices and post-authorization surveillance systems (French translation)
Watch video•Contact information

Expedited Regulatory Pathways – possible in developing countries with strong reliance practices and post-authorization surveillance systems (Spanish translation)
Watch video•Contact information

Bioequivalencia - Sistema de Clasificación Biofarmacéutica: Fundamentos y aplicaciones a las bioexenciones
Watch video•Contact information

Managing workforce demand in a pandemic – now and in future
Watch video•Contact information

Pharmacy Education Journal (PEJ): 20 years celebration event
Watch video•Contact information

‘How can digital health support national pharmaceutical care delivery?’ A regional and global assessment of priorities and challenges in the African region
Watch video•Contact information
The management of constipation by community pharmacists
Watch video•Contact information


Ensuring quality of care and patient safety through continuing professional development
Watch video•Contact information

The role of pharmacists in digestive wellness: an overview
Watch video•Contact information

Evidence and impact: where are we now and where next for data and intelligence for advancing pharmacy worldwide?
Watch video•Contact information
FIP Hospital Pharmacy Section Strategy Update
Watch video•Contact information

Technology and artificial intelligence in pharmaceutical education
Watch video•Contact information

The role of pharmacists in oral health
Watch video•Contact information

Pharmacists for the Future Programme by Curriculo Solutions: Changing the narrative for pharmacy leadership development. An FIP Seal-awarded programme
Watch video•Contact information

Therapeutic advances against COVID-19
Watch video•Contact information
Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults
Watch video•Contact information

The role of community pharmacies in COVID-19 booster vaccination: Opportunities and supply chain challenges in a post-pandemic scenario
Watch video•Contact information

Advocating for the role of pharmacists in diphtheria-tetanus-pertussis booster, COVID-19, and meningococcal meningitis vaccinations
Watch video•Contact information

Precision Medicine: The current status
Watch video•Contact information
Pharmacy school management for the shifts towards remote learning and virtual pharmacy education
Watch video•Contact information

COVID-19: where are we now and where next?
Watch video•Contact information

Epilepsy: Pharmacists and a Public Health Imperative
Watch video•Contact information

FDA (CDER): Generic Development and Bridging Global Regulations
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Eastern Mediterranean Region
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Southeast Asian Region
Watch video•Contact information

Professional Development and Advanced Training Opportunities for Pharmacy Technicians
Watch video•Contact information

Facilitar el acceso a los medicamentos: Bioequivalencia - Perspectiva de las agencias reguladoras de América Latina
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: African Region
Watch video•Contact information

Permettre la prescription et l'administration multiprofessionnelles des vaccins pour améliorer les taux de prise en charge: Région africaine
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: European Region
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Americas Region
Watch video•Contact information

Permitir la prescripción y administración multiprofesional de vacunas para mejorar las tasas de vacunación: Región de las Américas
Watch video•Contact information

Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Western Pacific Region
Watch video•Contact information

Empowering self-care through pharmacy – Guidelines and resources for pharmacists
Watch video•Contact information

FIP CPS Champion for Change 2021: How can pharmacists better help patients with mental health problems?
Watch video• Website•Contact information
Combatting Malaria: New horizon of vaccines and drug discovery by young pharmaceutical scientists
Watch video•Contact information

Innovation in cancer prevention, screening and management in community and hospital pharmacy
Watch video•Contact information

In Vitro In Vivo (IVIV) Correlations: Establishing a correlation between in vitro tests and in vivo product performance
Watch video•Contact information

FIP Train the Trainer online course for educators on digital health - Capitalizing on the benefits of digital health
Watch video•Contact information

Medication review and medicines use review services - a new toolkit for pharmacists
Watch video•Contact information

Mental health and resilience in higher education: Cultivating and sustaining wellbeing of the academics
Watch video•Contact information

Supporting self-care: sexual health
Watch video•Contact information

FIP Development Goals Special Event: Launch of a global report and new online knowledge centre
Watch video•Contact information
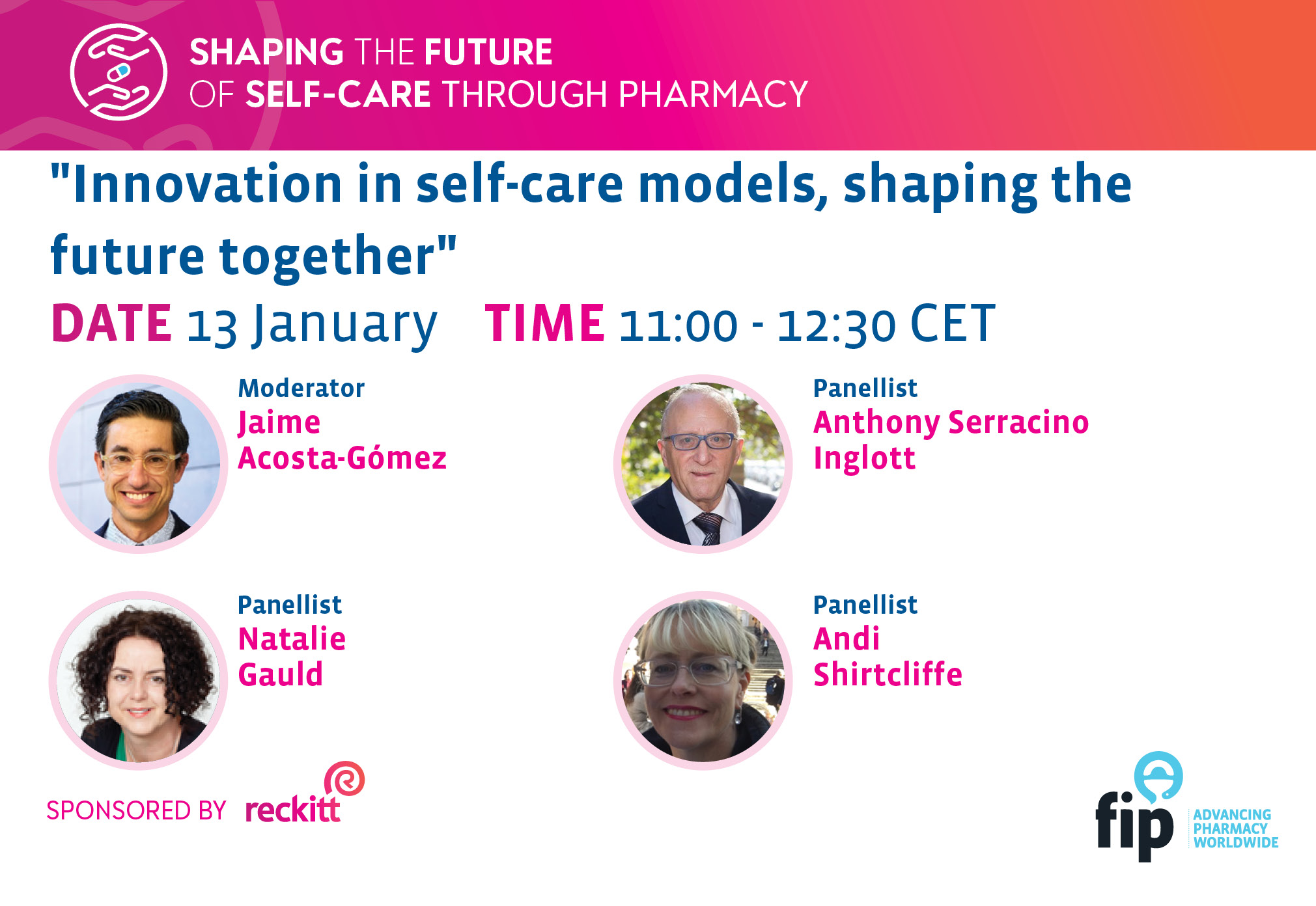
Innovation in self-care models, shaping the future together.
Watch video•Contact information

Supporting self-care: children's health
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 21 - FINALE | FIP DG 11: Impact & outcomes
Watch video• Contact •Contact information

Global frameworks to support career development
Watch video•Contact information

FIP-UNITWIN Global Summit on Pharmaceutical Education
Watch video• Contact •Contact information

FIP “Setting goals for the decade ahead” | Episode 20 | FIP DG 14: Medicines expertise
Watch video• Contact •Contact information

Self-care’s contribution to universal health coverage – Healthcare’s and people’s perspectives
Watch video•Contact information

Online pharmacy operations and distribution of medicines
Watch video•Contact information

Supporting self-care: Gastrointestinal complaints and treatments
Watch video•Contact information

The role of pharmacists in the prevention, screening and treatment management of HIV: An international update
Watch video•Contact information

Facilitar el acceso a los medicamentos: estudios de casos de reglamentación de bioexenciones de América Latina
Watch video•Contact information

Accelerating AMR Action Through Antimicrobial Stewardship: Are pharmacists the protagonists in the fight against antibiotic resistance?
Watch video•Contact information

Accelerating AMR action through antimicrobial stewardship in the South East Asian region
Watch video•Contact information

Accelerating AMR action through antimicrobial stewardship in the Eastern Mediterranean region
Watch video•Contact information

Accelerating AMR action through antimicrobial stewardship in the European region
Watch video•Contact information

Accelerating AMR action through antimicrobial stewardship in the Western Pacific region
Watch video•Contact information

Accelerating AMR action through antimicrobial stewardship in the African region
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 19 | FIP DG 17: Antimicrobial stewardship
Watch video• Contact •Contact information

Accelerating AMR action through antimicrobial stewardship in the Americas region
Watch video•Contact information

Supporting self-care: Sore throat
Watch video•Contact information
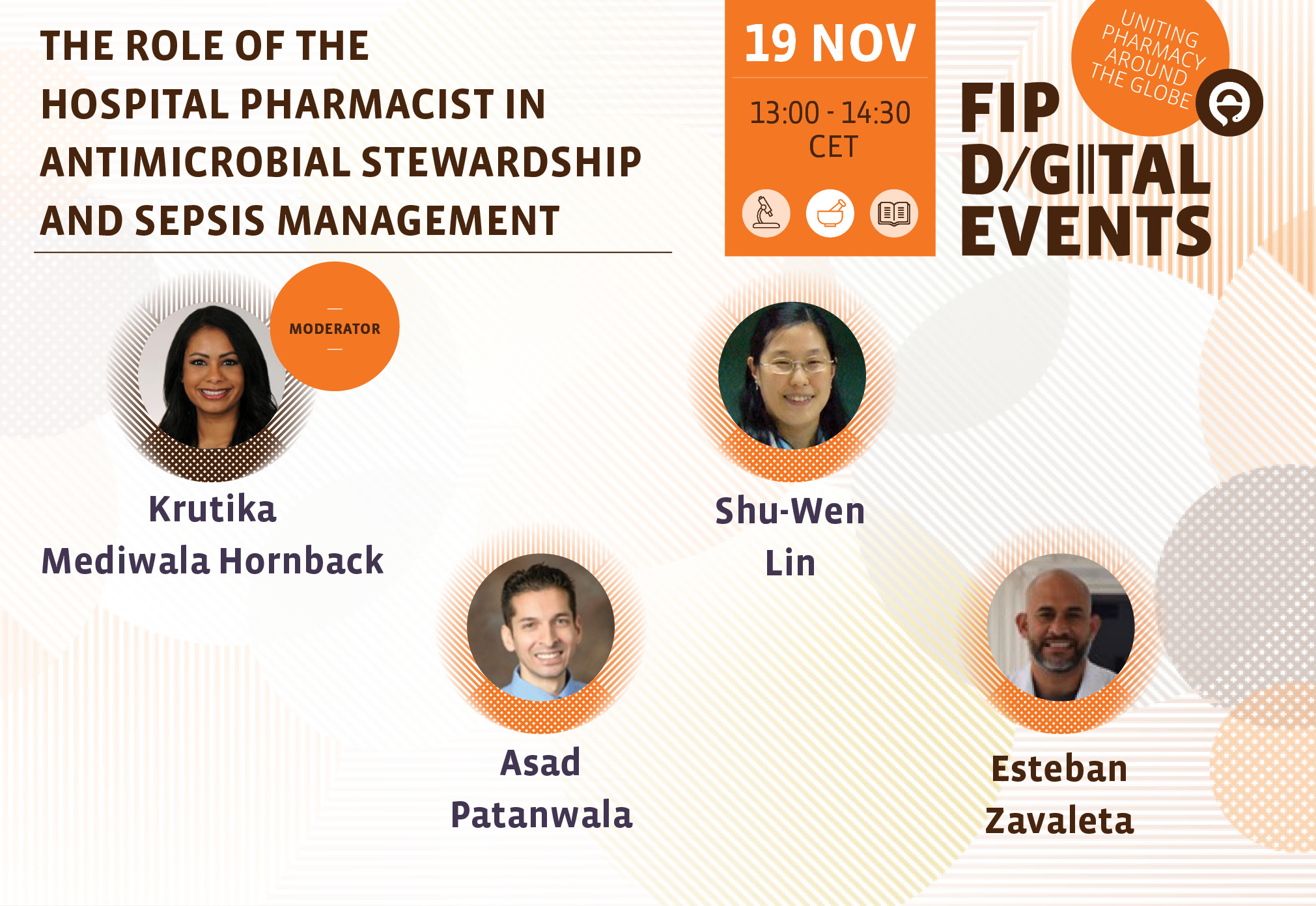
Role of the hospital pharmacist in antimicrobial stewardship and sepsis management
Watch video•Contact information

Leadership summit on pharmacy-based vaccination policy and advocacy
Watch video•Contact information

Uncovering our implicit biases to advance patient care: An introduction for pharmacists
Watch video•Contact information

Presentación del documento técnico Servicios farmacéuticos en inmunización: aportes, experiencias e implementación en la región de las Américas.
Watch video•Contact information

COVID-19 Complications – What Should Pharmacists Know?
Watch video•Contact information
Transforming pharmacy practice for improved care and management of diabetes
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 18 | FIP DG 3: Quality assurance
Watch video• Contact •Contact information

What technology can bring to cytotoxic drugs compounding
Watch video•Contact information

Competencies and workforce development strategies for diabetes related roles
Watch video•Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the African region
Watch video•Contact information

Diabetes therapeutics, treatment optimisation and support technology
Watch video•Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Western Pacific region
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 17 | FIP DG 5: Competency development
Watch video• Contact •Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the South East Asian region
Watch video•Contact information

3D printing of pharmaceuticals: get ready
Watch video•Contact information

FIP Global Summit on Primary Health Care: Pharmacists transforming vision into actions
Watch video•Contact information
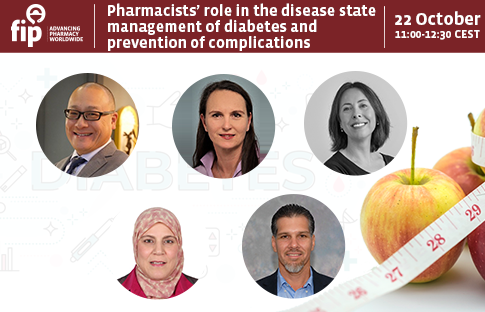
Pharmacists’ role in the disease state management of diabetes and prevention of complications
Watch video•Contact information

Joining forces across civil society organisations towards improved vaccination coverage
Watch video•Contact information

9th FIP Global Pharmacy Technicians’ Symposium_Day 2
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 16 | FIP DG 8: Working with others
Watch video• Contact •Contact information

9th FIP Global Pharmacy Technicians’ Symposium_Day 1
Watch video•Contact information

Career Paths and Opportunities in pharmacy and pharmaceutical sciences
Watch video•Contact information

Supporting self-care: disinfection in common conditions
Watch video•Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the European region
Watch video•Contact information
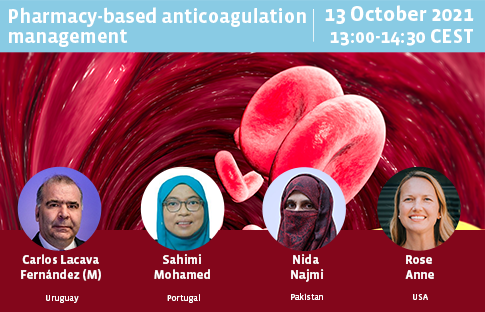
Pharmacy-based anticoagulation management
Watch video•Contact information
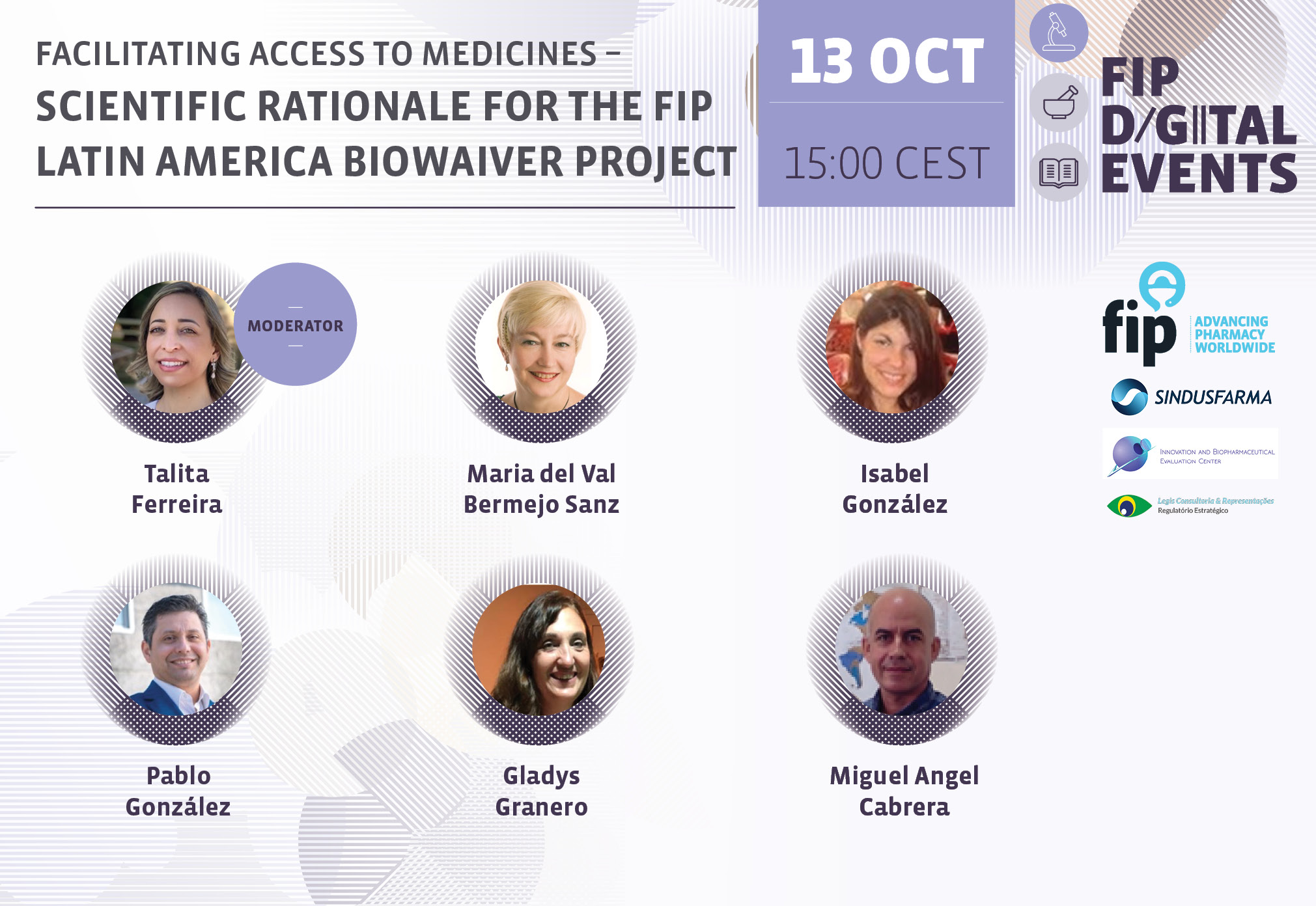
Facilitating Access to Medicines – Scientific Rationale for the FIP Latin America Biowaiver Project
Watch video•Contact information

FIP WiSE: Women in science and education: Launch of the enabling workplace toolkit
Watch video•Contact information

Confidence, complacency and convenience: Key elements of influenza vaccination strategies in times of COVID-19
Watch video•Contact information

Technology and new logistics – a disruption or a forced opportunity for community pharmacies
Watch video•Contact information

The role of pharmacists in the prevention of diabetes
Watch video•Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Eastern Mediterranean region
Watch video•Contact information

Accelerating selfcare to achieve Universal Health Coverage
Watch video•Contact information

Pharmacist-led mental health care during the COVID-19 pandemic and beyond
Watch video•Contact information

Digital Health and hospital Pharmacy
Watch video•Contact information

Acceso sostenible y equitativo a las vacunas: Establecimiento de prioridades y fijación de políticas en la región de las Américas
Watch video•Contact information

Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Americas region
Watch video•Contact information

mRNA Technology for Vaccines & Therapeutics
Watch video•Contact information

Pharmaceutical care for people with hearing impairment or deaf
Watch video•Contact information

Trusted for health- Pharmacy, Medication Adherence & Communication
Watch video•Contact information

Technology Forum Webinar on Virtual Reality
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 15 | FIP DG 12: Pharmacy intelligence
Watch video• Contact •Contact information

Working together across systems to transform vaccination policy: working with others in our professions, with other disciplines and agencies to establish sustainable policies
Watch video•Contact information

Trusted for health: Pharmacy and Counterfeit Medicines worldwide- problems and solutions
Watch video•Contact information

The role of the pharmacist in the prevention and management of vector-borne diseases: European region
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 14 | FIP DG 13: Policy development
Watch video• Contact •Contact information

FIP “Setting goals for the decade ahead” | Episode 13 | FIP DG 19: Patient safety
Watch video• Contact •Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the Southeast Asia region
Watch video•Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the Eastern Mediterranean Region
Watch video•Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the Europe region
Watch video•Contact information

Host Madsen Medal winner 2021 - Multifunctional Envelope-type Nano Device from Controlled Intracellular Trafficking to Clinical Application for Nanomedicines
Watch video•Contact information

Introducing the International Pharmaceutical Federation (FIP) and FIP Young Pharmacists’ Group (FIP YPG): A special event for pharmacists in Bahrain
Watch video•Contact information

Strategies towards improved patient outcomes with OTC analgesics
Watch video•Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the African region
Watch video•Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the Western Pacific region
Watch video•Contact information

Understanding and managing headaches in the community: Therapeutic approaches and advancements in the Americas
Watch video•Contact information

Suicide prevention, burnout and pandemics: Supporting pharmacists' mental health and wellbeing
Watch video•Contact information

Supporting self-care: Women's health
Watch video•Contact information

Apoyar el autocuidado: La salud de la mujer
Watch video•Contact information

Поддержка заботы о себе: Женское здоровье
Watch video•Contact information

Frauengesundheit: Selbstfürsorge stärken
Watch video•Contact information

Sostenere la cura di sé: la salute delle donne
Watch video•Contact information

Comprender la reticencia a las vacunas y fomentar la confianza en ellas mediante conversaciones eficaces
Watch video•Contact information

Understanding vaccine hesitancy and building vaccine confidence through effective conversations
Watch video•Contact information

Comprendre la réticence à l'égard de la vaccination et renforcer la confiance dans les vaccins par des conversations efficaces
Watch video•Contact information

Leveraging pharmacists to minimise the impact of air pollution on health: Policy barriers and drivers
Watch video•Contact information

Understanding and managing 21st century headaches in the community
Watch video•Contact information

Dynamics shaping the future self-care market
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 12 | FIP DG 2: Early career training strategy
Watch video• Contact •Contact information

Optimising opioid use through pharmacists
Watch video•Contact information

Strategies for a successful career change
Watch video•Contact information

Advanced and specialist pharmacist development for hospital pharmacists
Watch video•Contact information

YPG & IPSF: International Professional Organizations for Early Career Pharmacists & Pharmaceutical Scientists
Watch video•Contact information

Health illiteracy and vaccine misinformation as determinants for equity: developing policies to establish access to quality information in an equitable way
Watch video•Contact information

JoPPP-EMRO Pharm Forum Conference 2021 “Transformation of pharmacy practice towards patient care during the COVID-19 era” DAY 2
Watch video•Contact information

JoPPP-EMRO Pharm Forum Conference 2021 “Transformation of pharmacy practice towards patient care during the COVID-19 era” DAY 1
Watch video•Contact information

The pharmacist in the prevention and management of vector-borne diseases: a perspective from the Southeast Asian region
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 11 | FIP DG 7: Advancing integrated services
Watch video• Contact •Contact information

Community pharmacy roles, services and tools to minimise impact of air pollution on health
Watch video•Contact information

Selfcare in the digital age
Watch video•Contact information

Vaccinations and the genders: Examining inequities in gender access and handling of vaccinations globally to inform pharmacy policy
Watch video•Contact information

Role of Early Career Pharmaceutical Organisation (ECPO) in Global Health
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 10 | FIP DG 4: Advanced and specialist development
Watch video• Contact •Contact information

Pharmacists’ experiences in crises in Lebanon: The Lebanese financial crisis, COVID-19 pandemic, and the Beirut Port blast.
Watch video•Contact information

Supporting self-care: Footcare in diabetes management
Watch video•Contact information

Developing Effective Pharmacy-led Vaccination Campaigns
Watch video•Contact information

Développer des campagnes de vaccination efficaces par les pharmaciens
Le webinaire "Développer des campagnes de vaccination efficaces par les pharmaciens" a présenté les stratégies pour développer ces campagnes afin de communiquer la valeur des vaccins à des groupes de patients spécifiques, de renforcer la confiance et l’adoption de la vaccination.
MoreContact information

Desarrollo de campañas de vacunación eficaces por las farmacias
El seminario web " Desarrollo de campañas de vacunación eficaces por las farmacias” ha presentado estrategias robustas para el desarrollo de campañas de inmunización por parte de farmacéuticos con el fin de comunicar el valor de las vacunas a grupos específicos de pacientes, crear confianza en las vacunas y mejorar su aceptación.
MoreContact information

Readying the global population for self-care: Health literacy in the 2020s
Watch video•Contact information

The role of pharmacists in the prevention and management of vector-borne diseases: a perspective from the Western Pacific region
Watch video•Contact information

O papel do farmacêutico nas doenças não transmissíveis / The role of pharmacists in non-communicable diseases
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 9 | FIP DG 6: Leadership development
Watch video• Contact •Contact information

Effectively developing transferable skills and the positive impact of training and certification in career progression
Watch video• Website•Contact information

Delivering asthma right care: For hospital pharmacists
Watch video•Contact information

How regulations impact pharmacy technician practice
Watch video•Contact information

"Just Culture" approach to community pharmacy practice regulation - An overview
Watch video•Contact information

Looking ahead: Post-pandemic recovery and restoration of services
Watch video•Contact information

Educating a contemporary hospital pharmacy workforce and continuing their professional competence in developing countries
Watch video•Contact information

Equity, access & sustainability through life’s ages and stages: Enabling a life course-approach to vaccination
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 8 | FIP DG 15: People-centred care
Watch video• Contact •Contact information

Pharmacists in long-term care facilities: Before, during and after the pandemic
Watch video•Contact information

Delivering asthma right care: For community pharmacists
Watch video•Contact information

Setting up new FIP UNITWIN centres for excellence and establishing pharmacy schools’ associations: Launch of the FIP UNITWIN pathfinder toolkit
Watch video•Contact information

Supporting self-care: musculoskeletal pain and chronicity
Watch video•Contact information

Boas Práticas de Farmácia /Good Pharmacy Practice
Watch video•Contact information

Joining forces across health professionals towards improved vaccination coverage
Watch video•Contact information


Pharmacy Practice Research Virtual Summer Meeting for PhD students, Postdoctoral fellows and supervisors
Watch video•Contact information

Workshop on systematic reviews and meta-analyses
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 7 | FIP DG 9: Continuing professional development strategies
Watch video• Contact •Contact information

Distribution of medicines and promotion of responsible self-care: Channels, changes and challenges
Watch video•Contact information

The role of pharmacists in the prevention and management of vector-borne diseases: a perspective from the Eastern Mediterranean region
Watch video•Contact information

Introducing the FIP ‘Transforming Vaccination Globally, Regional and Nationally’ 2021: Accelerating equity, access and sustainability through policy development and implementation
Watch video•Contact information

Delivering person-centred support for self-care: current and future pharmacy practice
Watch video•Contact information

Como começar uma organização de Jovens Farmacêuticos em início de carreira/ How to start an association of young pharmacists in start of career
Watch video•Contact information

Outdoor and indoor air pollution: short- and long-term impacts on health
Watch video•Contact information

Mentorship: Effectively utilising the GROW model as a way to develop your skills and expertise.
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 6 | FIP DG 21: Sustainability in pharmacy
Watch video• Contact •Contact information

FIP Guidelines for Dissolution Testing of Solid Oral Products
Watch video•Contact information

If you knew then what you know now: Sharing and assessing one year of remote and online learning
Watch video•Contact information

Obesity and weight management: Pharmacist-led services and approaches
Watch video•Contact information

Drugs to tackle a pandemic – something old and something new
Watch video•Contact information

Gestión adecuada de la selección de medicamentos en autocuidado
Contact information

Appropriate management in the choice of medicines for self-care
Watch video•Contact information

La farmacia humanitaria durante el covid-19. Gestión de desafíos durante una nueva era
Watch video• Website•Contact information

Nutritional approaches to the prevention and management of non-communicable diseases: Roles for pharmacists
Watch video•Contact information

Community pharmacy regulation, access and remuneration – global trends
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 5 | FIP DG 1: Academic capacity
Watch video•Contact information

Medicamentos Falsificados e Sub-standard / Falsified and substandard medicines
Watch video•Contact information

The role of the pharmacist in the prevention and management of vector-borne diseases: African region
Watch video•Contact information

Shaping the future of self-care: healthcare needs & challenges
Watch video•Contact information

Hearing from our heroes: Students and young pharmacists on the frontline Part 2
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 4 | FIP DG 20: Digital health
Watch video• Contact •Contact information

QbD in Biologics Drug Product Development and Manufacturing
Watch video•Contact information

Virtual delivery in class: Spicing up lectures in an online environment and maintaining the quality and standards as an academic pharmacy leader
Watch video•Contact information

Digital health in pharmacy education: Global showcase of initiatives from pharmacy schools
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 3 | FIP DG 16: Communicable diseases
Watch video•Contact information

Doenças transmitidas por vetores /Vector-borne diseases
Watch video•Contact information

El farmacéutico en la prevención y manejo de las enfermedades transmisibles por vectores: una perspectiva de la región de las Américas
Watch video•Contact information

Innovative cultural sensitivity training for pharmacy students
Watch video•Contact information

Medicines reconciliation: an essential pharmacist-led service to ensuring patient safety
Watch video•Contact information

Pharmacy technician research – highlighting recent literature
Watch video•Contact information

Reducing medication harm through medicines use reviews: pharmacists at the frontline
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 2 | FIP DG 18: Access to medicines, devices & services
Watch video•Contact information

Online access to medicines: lessons learnt for self-care
Watch video•Contact information

Continuing Professional development cycle; a suitable career development model for early career pharmacists and pharmaceutical scientists
Watch video•Contact information

Online pharmacy operations around the world
Watch video•Contact information

FIP “Setting goals for the decade ahead” | Episode 1 | FIP DG 10: Equity & equality
Watch video•Contact information

Generic Drug Global Landscape - An FDA Perspective
Watch video•Contact information

Navigating an academic career in the field of pharmaceutical sciences
Watch video•Contact information

Career development: What a career at a pharmaceutical company might look like
Watch video•Contact information

Increasing enrolment in higher education to strengthen and improve the pharmacy profession
Watch video•Contact information
Pharmacists' involvement in COVID-19 vaccination: Addressing regulatory needs
Watch video•Contact information

The role of the pharmaceutical scientist in a government body
Watch video• Contact •Contact information
Address: Online

Preceptor Pearls for Hospital Pharmacists
Watch video•Contact information

Global Launch of the FIP digital health in pharmacy education report: Developing a digitally enabled pharmaceutical workforce
Watch video•Contact information

Manufacturing Classification System for oral solid dosage forms: Good in Theory and in Practice
Watch video•Contact information
Global Immunization Initiatives: Implementing Lifelong Learning to Address Mass Vaccination
Watch video•Contact information

The pharmacy practice research journey – Moving from your research idea to publication
Watch video•Contact information

Vaccine administration routes and procedures: frequent concerns and common errors
Watch video•Contact information

FIP Global Virtual Summit: Committing to action to transform vaccination in pharmacy
Watch video•Contact information

From regional to global: Common priorities for action to transform vaccination
Watch video•Contact information

Global Launch of the FIP YPG Career Development Toolkit for Early Career Pharmacists and Pharmaceutical Scientists
Watch video•Contact information

Resiliency in the face of COVID-19 – Pharmacy technicians rise to the global crisis
Watch video•Contact information

Necesidades e impulsores regionales de la transformación de la vacunación: Américas
Watch video•Contact information

Regional needs and drivers for transforming vaccination: Africa
Watch video•Contact information

Qualification of dissolution apparatus
Watch video•Contact information

Regional needs and drivers for transforming vaccination: Western Pacific
Watch video•Contact information

Unleashing resilience in pharmacy: global perspectives
Watch video•Contact information

Regional needs and drivers for transforming vaccination: Eastern Mediterranean
Watch video•Contact information

Regional needs and drivers for transforming vaccination: South East Asia
Watch video•Contact information

Regional needs and drivers for transforming vaccination: Europe
Watch video•Contact information

Communicating vaccine safety, building vaccine confidence
Watch video•Contact information

Setting transformative goals for vaccination globally
Watch video•Contact information
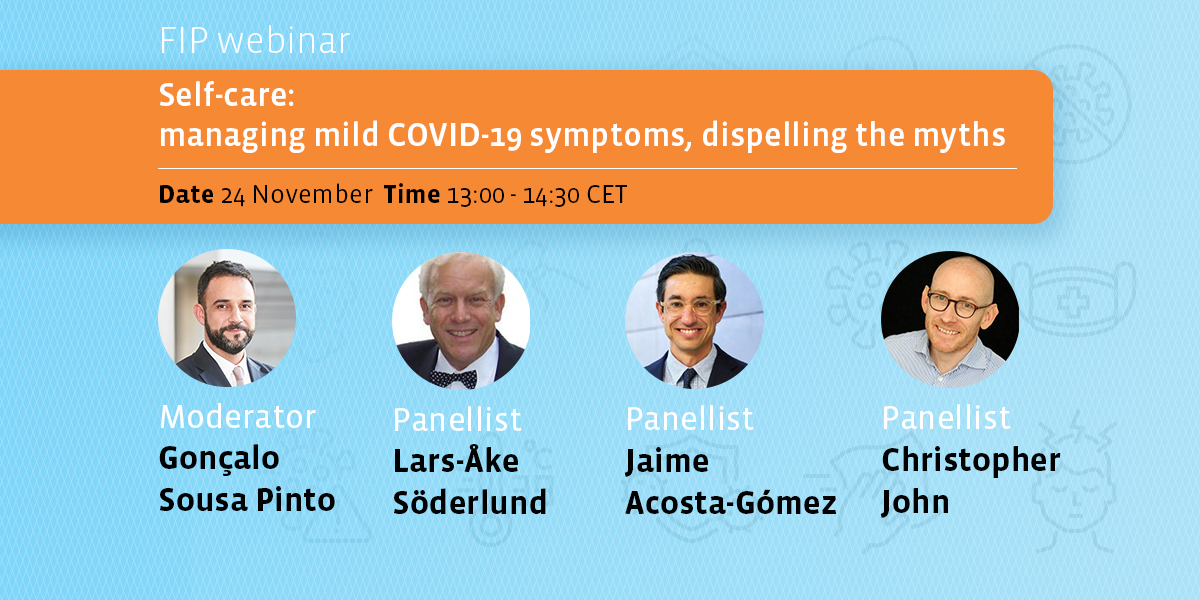
Self-care: managing mild COVID-19 symptoms, dispelling the myths
Watch video•Contact information

The Role of the Pharmacist in the Care of Obstetric Patients
Watch video•Contact information
COVID-19 Guidance on Evaluation of Diagnostic Testing & Methods
Watch video•Contact information

Pharmacist vaccinators and patient safety: FIP DG 19 Patient Safety
Watch video•Contact information

The Role of Pharmacist Vaccinators in improving access to and equity in healthcare outcomes: FIP DG 10 Equity & equality and FIP DG18 Access to Medicines & Services
Watch video•Contact information
2020 FIP survey on community pharmacy, self-care, and digital and online pharmacy operations
Watch video•Contact information

Covid-19: toma de decisiones en ausencia de evidencia científica. Farmacovigilancia y tecnovigilancia como herramientas para evaluación de riesgo
Watch video•Contact information

Pharmacist vaccinators and antimicrobial stewardship: FIP DG 17 Antimicrobial Stewardship
Watch video•Contact information
Pharmacist vaccinators and communicable disease management: FIP DG 16 Communicable Diseases
Watch video•Contact information

COVID-19 and the pharmacy technician response to a rapidly shifting healthcare landscape
Watch video•Contact information

Removing policy barriers to pharmacist vaccinations: FIP DG 13 Policy Development
Watch video•Contact information

Pharmacy Practice Research Showcase
Watch video•Contact information

Empowering pharmacists to deliver vaccination at the health-system level: FIP DG 7 Advancing Integrated Services
Watch video•Contact information

Vaccination from specialist practice to every day practice: FIP DG 4 Advanced & Specialist Development
Watch video•Contact information

CPS Champions for Change webinar
Watch video•Contact information

L'hiver arrive : La vaccination contre la grippe en période de COVID-19. Bonnes pratiques des pays francophones
Watch video•Contact information

Reviewing the FIP Transforming Vaccination Programme: Enabling and supporting our profession
Watch video•Contact information

Global Launch of the FIP Pharmacy Education in sub-Saharan Africa Report: A decade of education partnership across Africa
Watch video•Contact information

Enabling our workforce: Supporting ongoing competence
Moderators: Ralph Altiere and Ian Bates. Panellists: Omotayo Carolyn Olaoye, Mitch Rothholz and Han Zhe.
MoreContact information

Transforming our workforce: Evolving the pharmacist's qualification
Watch video•Contact information

Medicines use and quality
Chair: Paul Sinclair. Speakers: Tamara Peiró Zorrilla, Jan Saevels and Jephney John Redford Jacquet
Contact information

Shaping The Future Of The Pharmacy Profession Through Pharmacy Practice Research?
Moderator: Fernanda Stumpf Tonin. Panellists: Martin Schulz, Charlie Benrimoj and Victoria Garcia Cardenas
MoreContact information
Tips for using the Basel Statements Self-assessment Tool
Watch video•Contact information

Avances en el desarrollo de vacunas contra COVID-19 e importancia de la vacunación en tiempos de pandemia.
Watch video•Contact information

Medicines shortages mitigation and waste optimization
Watch video•Contact information

From smallpox to COVID-19: Vaccine development & innovation
Watch video•Contact information

Addressing barriers to uptake: Adherence, misinformation & anti-Science
Watch video•Contact information

Enabling practice: Empowering pharmacists & removing barriers
Moderators: Ema Paulino, Paul Sinclair. Panellists: Dalia Dawoud, Helena Rosado, Sofía Segura.
Contact information

Transforming practice: A focus on strategy & policy for global change
Watch video•Contact information

A global approach to pharmacy workforce development: Launch of the FIP Workforce Reference Guide
Watch video•Contact information

SIGN 2 – Pharmacists’ roles in NCDs, healthy lives and disease prevention
Watch video•Contact information

COVID-19 Complex Case Presentations
REGISTER HERE!
MoreContact information

Introducing the FIP 'Transforming Vaccination Globally & Regionally' series Programme… needs, action and outcomes
Contact information

Launch of FIP Commission on antimicrobial resistance (AMR)
Watch video•Contact information

FIP Development Goal 10: Equity & equality for pharmacy now
Watch video•Contact information

FIP-WiSE – Women’s Leadership Lab – Web Panel
Watch video•Contact information
FIP Sharing Information in a Global Network (SIGN) Session: Workforce development and transformation strategies
Watch video•Contact information

Transforming Global Pharmacy: Launch of the FIP Development Goals
Watch video•Contact information

Northern Ireland Pharmacy Response
Watch video•Contact information

The value of vaccines for society and special populations
Watch video•Contact information

Influenza vaccination – Strategic elements of development, supply and delivery for optimal prevention
Watch video•Contact information

Can the world afford low vaccination coverage rates? Broadening vaccination gateways through pharmacies
Watch video•Contact information

The impact of air pollution on respiratory health and vulnerability to COVID-19. What can community pharmacists do to help?
Watch video•Contact information

Driving AMR action in a new decade
Watch video•Contact information

The immediate global impact of COVID-19 on higher education institutions & workforce development
Moderator: Jill Boone, Pierre Moreau. Panellists: Ian Bates, Louise Brown, Shepard Mhalaba, Mariet Eksteen and Vibhuti Arya.
MoreContact information

Give it a shot: Advocating for pharmacy-based vaccination and achieving legislative changes
Moderator: Paul Sinclair. Panellists: Lisa Nissen, Carine Wolf-Thal and David Sinclair.
MoreContact information

Ask the Presidents: Reflections on COVID-19 biggest impact, concerns and opportunities in the post-pandemic world
Moderator: Catherine Duggan. Panellists: Dominique Jordan, Robert Moss, Lars-Åke Söderlund and Ulf Janzon.
Contact information

Pharmacy Practice Research priorities during the COVID-19 pandemic
Moderators: Charlotte Verner Rossing and Dalia Dawoud. Panellists: Shane Desselle, Ian Bates and Zaheer Babar.
MoreContact information

Efectos del COVID-19 en la salud de los profesionales sanitarios y estrategias futuras de prevención
Watch video•Contact information

An overview of current pharmacy impact on immunisation – Presentation of key findings from FIP's global report 2020
Facilitator: Lars-Åke Söderlund. Panellist: Gonçalo Sousa Pinto.
MoreContact information

Addressing inequities in pharmacy education due to COVID-19 – Learnings from Africa, Asia and Latin America
Moderator: Ralph Altiere. Facilitator: Nilhan Uzman. Panellists: Bhojraj Suresh, Derick Munkombwe, Alison Williams, Patricia Acuna-Johnson, Nguyen Van Hung and Jennie Lates.
MoreContact information

The regulatory climate with COVID-19
Moderator: Timothy Chen.
Facilitator: Tara Hehir.
Panellists: Cody Midlam, Peter Guthrey, Carolina Ung and Betty Chaar
Contact information
Ready, set, go! The role of the pharmacist in doping control in sports
Moderator: Kerstin Wagner. Panellists: Daniel Sanabria, Mark Stuart and Helen Zhang.
MoreContact information

Evidence-based practice during the COVID-19 pandemic
Moderator: Victoria Garcia Cardenas. Panellists: Filipa Alves da Costa, Fernanda Stumpf Tonin and Dalia Dawoud.
MoreContact information
Creativity in pharmacy education
Moderator: Ruth Edwards. Facilitator: Nilhan Uzman. Panellists: Richard Silvia, Maria Allinson, Amrita Multani and Sara Ibrahim.
MoreContact information

Youth involvement in policy research + advocacy: Creating policies at a global level of FIP + IPSF
Panellists: Hera Ali and Zuzana Kusynová.
MoreContact information

The Rise of Substandard & Falsified Medical Products during the COVID-19 (Part2)
Moderator: Lina Bader. Panellists:Oksana Pyzik, Maryam Jetha, Mike Isles, Flandrie Habyarimana and Stanislas Barro.
Contact information

Increasing vaccination coverage through pharmacists | Episode 2: Who’s immune to fake news? Addressing patient motivation and vaccine hesitancy
Moderator: Ema Paulino. Panellists: Jane Barratt, Caterina Suitner, Darragh O’Loughlin
MoreContact information

Medicine supply disruptions and shortages during the pandemic
Moderator: Ulf Janzon. Panellists: Propser Hiag, Joan Alexis, Joao Ferreira, J. Jayaseelan. Facilitators: Clement Haeck, Gerard Lee See
MoreContact information
Address: Online

Return to Pharmacy Education: Planning for the Upcoming Academic Year
Moderator: Pierre Moreau. Facilitator: Ozge Ozer. Panellists: Lilian M. Azzopardi, Paul Gallagher, Mohamad Rahal, Yulia Ladutko.
MoreContact information

Remote Laboratory Courses Across Pharmacy Schools During COVID-19: Are You Ready?
Moderator: Dalal Hammoudi. Panellists: Malaika Turner, Indiran Pather, Peter J Rice, Susan Finstrom, Tom Anchordoquy, Edmund Ekuadzi, Chelsea Baker, Jamie L. Woodyard, Susana Abdel Fattah, Rana Mohaidly and Nicolette Sammut Bartolo.
MoreContact information
Intro to FIP SIG: Drug Delivery & Manufacturing, New Medicines & New Generation of Pharm. Scientists
Panellists: Aliasger K. Salem, Takuya Kumamoto and Rebecka Isaksson
Contact information

Key considerations for developing COVID-19 treatments: learning from the past and planning for the future
Watch video• Contact •Contact information
Green and sustainable pharmacy practice – Guidance for practitioners
Speakers: Eeva Teräsalmi and Jaakko Teppo
MoreContact information

Winter is coming: Influenza vaccination in times of COVID19–Best practices from Southern Hemisphere:
Panellists: Mariet Eksteen, Bettina Caponi and Andi Shirtcliffe.
MoreContact information

MyDispense: A virtual simulation to teach pharmacy students across the globe
Panellists: Clark Kebodeaux, Lisa Holle, Jill Fitzgerald, Sarah Vordenberg, Pui San Saw and Marian Costelloe
MoreContact information

#AfricaTogether Pharmacy against COVID-19
Panellists: Jean-Baptiste, Barbara Nel, Khadija Jamaloodien, Jocelyn Chaibva, Daniella Munene, Jackie Maimin, Benjamin Kwame Botwe and Nuvin Juggessur.
Contact information

COVID-19 for hospital pharmacists and health-systems: The medication use process
Panellists: Ana Herranz, Michael Ganio, Adenjii Emmanuel Adefemi and, Nai-Hwa Mei
MoreContact information

The FIP CEO Interviews... Trevor Jones
Dr Duggan’s first guest is Trevor Jones, visiting professor at King’s College London and former Head of Research and Development at Wellcome, UK.
MoreContact information

Good pharmacy practice during the COVID-19 pandemic
Speaker: Eeva Teräsalmi
MoreContact information

The rise of substandard & falsified medical products during the COVID-19 pandemic
Panellists: Oksana Pyzik, Maryam Jetha, Mike Isles, Flandrie Habyarimana and Stanislas Barro
Contact information

COVID-19: Women front and center.
Panellists: Mariam El Boakye-Gyasi, Ecehan Balta, Rajani Shakya and Anyango Esther.
Contact information

Challenging the narrative on leadership in gender equity during the COVID-19 pandemic
Watch video• Contact •Contact information
World pharmacists’ COVID-19 online meeting
Webinar organised by theTurkish Pharmacists’ Association (TPA), in collaboration with FIP.
Panellists: Ahmet Çakan, Jacqueline Surugue, Ash Soni, Eeva Teräsalmi and Jaime Acosta
Contact information

Hearing from our heroes: Students and young pharmacists on the frontline Part 1
Panellists: Abrar Husaini, Anas Najjar, Nalukwago Mercy Kamya, Ramon Contrucci, Sara Ali Isaa and Sunil Shrestha
Contact information

Impact of COVID-19 on pharmacy education: Perspective from students & academics
Panellists: Claire Anderson, Khalid Garba Mohammed, João Guedes, Dr Sarira El-Den.
Contact information

Part 2 Remote & online education during COVID 19 “How to” suggestions
Panellists: Vivienne Mak, Rebekah Moles, Sarah Scoular, Vibhu Solanki, Youness Karodeh and Daniel Moraga.
Contact information

Hearing from our heroes Pharmacists on the Frontline during the Pandemic in Pakistan
Panellists: Aasma Hamid, Bismah Nayyer, Laiq Khan, Alamgir Rao and Huma Rasheed
Contact information

Experiential education in trying times It can be done! Let’s explore how
Panellists: Kirstie Galbraith, Patricia Acuna-Johnson, Fouad Sakr, RPh, Ralph Altiere, Vibhu Solanki, PhD, Jamila Jorden, Claire Anderson, Kyle Wilby, Marwan Akel, Priscilla Owusu-Mensah, Caroline Ruth Weinstein-Oppenheimer and Simon Furletti
Contact information

Communicating COVID 19 risk to promote positive behaviour change
Panellists: Liesje Donkin, Stefanie Pügge, Carina Vetye and Amy Chan
Contact information
Young pharmacists and scientists at the helm of a pandemic showcasing innovations & collaboration
Panellists: Sylvester Adeyemi, Audrey Clarissa, Ahmad El Ouweini and Catarina Nobre.
Contact information
Hearing from our heroes: Mental health and resilience of the workforce
Panellists: Sarah Dineen-Griffin, Catriona Bradley, Iman Bashiti, Mariet Eksteen and Sunayana Shah.
Contact information
Pharmacy’s fight against COVID-19 in the USA
FIP Webcast with the American Society of Health-System Pharmacists.
Panellists: Arash Dabestani and Daniel Cobaugh
Contact information
Hearing from our heroes. Pharmacists fighting COVID-19 at the frontline
Panellists: Luna Al Bizri, Nadia Bukhari, Piotr Merks, Jaime Acosta Gomez and Nelly Nonette
Contact information
Comunicar COVID-19 desde la farmacia de comunidad. Comunicación en crisis
Panellist: Alejandra Fernández Jiménez
Contact information
Role of regulators in addressing the WHO Patient Safety Challenge
Panelists: Dan Burns, Catherine Duggan, Leonora O'Brien and Anastasia Shiamptanis
Contact information
Mass vaccination campaigns
Panellists: Ms Petra Straight and Mr Steve Shaddock.
Contact information
Remote & online education in trying times: Sharing and learning from each other
Panellists: Ralph Altiere, Carl Schneider, Nzeribe Ella, Daniel Moranga, Youness Karodeh, Jamila Jorden, La' Marcus Wingate, Vibhu Solanki, Claire Anderson, Shaun Gleason, Sarah Scoular, Rebekah Moles, Daniel Malone, VIVIENNE Mak, Patricia Acuna,
Contact information
Agua purificada para uso farmacéutico según OMS (Informe 47, Anexo 2)
Panelist: Dr. Francisco Javier Eguiguren
Contact information
¿Cuál es el aporte del farmacéutico asistencial en la pandemia por COVID-19?
Moderator: Nuria Montero. Panelists: Dra. Sofía Segura Cano.
Contact information
Experience Sharing of COVID-19 from China: Medicine & Pharmacist
Panellists: Rongsheng Zhao, Dong Liu, Yun Liao
Contact information
FIP guidance on COVID-19 for pharmacists and the pharmacy workforce
Panellist: Gonçalo Sousa Pinto
Contact information
FIP YPG Webinar: Introduction to FIP Sections and SIGs - A Focus on HaMIS, SAPS and PPR SIG
Panelists: Boyan Todorov, Timothy Chen and Victoria García Cardenas.
Contact information
Academic Section Webinar- Interprofessional Education_ The Pharmacists Role Across the Globe
Panelists: Cameil Wilson-Clarke, Gina Prescott, Lisa Hong, Toyin Tofade, Miranda Law.
Contact information

The contribution of pharmacists in non communicable diseases - Cancer
Panelists: Pep Guiu, Deirdre Criddle.
Contact information
FIP YPG grant for professional innovation - How to prepare a successful application
Speakers: Rebekah Moles, Filipa Ferreira and Jack Collins
Contact information

Navigating an academic career in the field of pharmaceutical sciences
Watch video•Contact information

The contribution of pharmacists in non-communicable diseases. Cardiovascular diseases
Panelist: Michael Hogue, Yetunde Oyeneyin.
Contact information
Coronavirus 2019-nCoV: How can pharmacists help control the outbreak?
Panellist: Gonçalo Sousa Pinto
Contact information
The contribution of pharmacists in non-communicable diseases: Asthma and COPD
Speakers: Sian Williams, Kristina Billberg.
Contact information

The contribution of pharmacists in non-communicable diseases: Diabetes
Speakers: Isabel Jacinto, Luna El Bizri
Contact information
The value and application of using health theories and models in pharmacy education and practice
Speakers: Hoai-An Troung, Miranda Law, Robbie Turner
Contact information

The role of youth in achieving one of the triple billion targets of the World Health Organization
Speakers: Caline Mattar (GHWN Youth Hub), Audrey Clarissa (Asian YPG), Aya Jamal (IPSF), and Diah Saminarsih (WHO)
Contact information

Targeted medication safety best practices for hospitals
Watch video• Contact •Contact information

Celebrating three years of Nanjing Conference: How young pharmacy workforce and students can be involved
Speakers: Lina Bader (FIP), Miranda Law (Howard University College of Pharmacy), and Alison Williams (IPSF)
MoreContact information

Advanced role of the next generation of pharmacists and pharmaceutical scientists in public health
Speakers: Clement Haeck and Tsai (Christopher Chua) Yu-Lin
Contact information
Good distribution practices. Securing the supply chain
Speaker: Michael H. Anisfeld
Contact information

Good storage & distribution. Pharma 4.0
Watch video• Website• Contact •Contact information
Digital health: Where does pharmacy stand?
Speakers: Peter Guthrey, Nada Moulla and Adebayo Alonge
Contact information
Culturally competent pharmacists: A key to patient-centered care
Speakers: Nisreen Mourad & Sally Arif
Contact information
International pharmacy accreditation
Speakers: Lynnae M Mahaney & Sherif Kamal
Contact information
FIP YPG-IPSF. Benefits and opportunities
Speakers: Petra Orlić & Diana Ching
Contact information
GALF stories. Persuasive leadership and how I developed and implemented the strategic plan for my faculty
Speakers: Ruth Edwards & Toyin tofade
Contact information
Health leaders united for change: Driving transformative gender equity policies for the global health workforce
Speakers: C. Duggan, A. Kelling, M. McIsaac, E. Paulino & B. S. Savic
Contact information
What leadership styles are suitable for academia
Speakers: Pierre Moreau and Toyin Tofade
Contact information
Tupaia project: A multi-country health systems mapping project for strengthening health supply chains
Speaker: Michael Nunan
Contact information
How FIP can help your organization
Speakers: John Pieper and Ema Paulino
Contact information
Pharmacists’ important role as a sexual health counsellor in the Gulf Countries
Speaker: Ian J. Cubbin
Contact information

Successfully engaging stakeholders to achieve needed and desired change
Speakers: Mike Rouse & Arman Üney
Contact information
Clostridium difficile infection management and outcomes. Considerations in a limited-resource setting
Speakers: Laurel Legenza & Renier Coetzee
Contact information
Innovations of IPE implementation
Speaker: Tina Brock
Contact information
Assessment of interprofessional education
Speaker: Sylvia Langlois
Contact information
Challenges of nanomedicine follow-on products. From regulatory to formulary selection
Speakers: Vinod Shah, Stefan Mühlebach & John Hertig
Contact information
Interprofessional education: Defining the competencies and goals
Speakers: Frank J. Ascione and Mariet Eksteen
Contact information
Pharmacist role in detection, control and prevention of arboviroses
Speaker: Cristina Fernández Barrantes
Contact information
Do it right for your patient: advantages and considerations associated with nanomedicines
Speakers: Gerrit Borchard and Jacqueline Surugue
Contact information
Participación del farmacéutico en la detección, control y prevención de las arbovirosis
Speaker: Cristina Fernández Barrantes
Contact information
Nanomedicines are different. But why?
Speakers: Jacqueline Surugue & Daan J.A. Crommelin
Contact information

What you need to know about the FIP Pharmaceutical Workforce Development Goals
Speakers: Ian Bates and Lina R. Bader
Contact information
Purposeful global engagement in pharmacy education
Speakers: Naser Alsharif, Miranda G. Law, Mona Schaalan & Prosper Mapo
Contact information
Formative assessment strategies in the different settings of pharmacy education (Part 1)
Speakers: Mohamad Rahal, Michelle Cherfan & La'Marcus T. Wingate
Contact information

Safe handling of hazardous drugs
Speakers: Stephen Eckel, Olga Delgado Sanchez and Jim Siderov
Contact information
The use of pictograms and infographics to support medication literacy and medication adherence
Speakers: Annie Pouliot, Régis Vaillancourt and Raul Malhotra
Contact information
Antimicrobial use and the value of stewardship globally
Speakers: Douglas Slain & Prosper Maposa
Contact information
Official launch - Report on the outcomes of global conference on pharmacy and pharmaceutical sciences education
Speakers: Philip Schneider and Ian Bates
Contact information
Hospital pharmacy automation internationally. Impact on patient safety
Speakers: Richard Caldwell, Maryanne Molenaar and Aygül Köseoğlu
Contact information
Poster and abstract preparation
Speakers: Rebekah Moles and Ryan A. Forrey
MoreContact information

Medication safety best practices
Speakers: John B. Hertig, Abdul Latif Sheikh, and Jacqueline Surugue
Contact information
Leadership development. For you and your faculty team!
Speakers: Jenelle Sobotka and Pierre Moreau
MoreContact information

Leadership development – for you and your students!
Speakers: Jenelle Sobotka and Pierre Moreau
MoreContact information

Counterfeit medications
Libby Baney and Nkechi Christiana Anyanwu
MoreContact information

Introduction to Pharmacoeconomics
A/Prof Dr. Ola Al Ahdab, Phd
Contact information

Basel Statements: a way to go forward
Jacqueline Surugue, Andy Gray