Export this Abstract | Print this Abstract | Add to my preferred Abstracts list | My preferred Abstracts list (0) | Back to Search
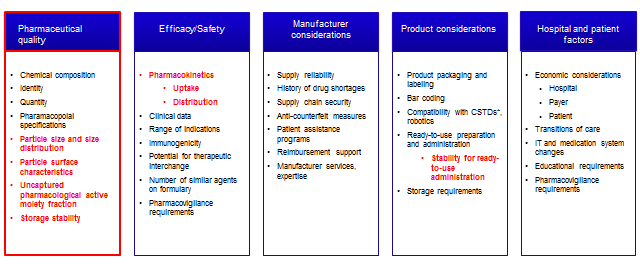 A systematic and objective approach to the selection of nanomedicines and nanosimilars for clinical use
A systematic and objective approach to the selection of nanomedicines and nanosimilars for clinical use
- In: Short Communications E2 on Tuesday, 23 May 2017, 11:15-12:30
- At: Stockholm (Sweden) (2017)
- Type: Presentation
- By: FLüHMANN, Beat (., Zürich, Switzerland)
- Co-author(s): Beat Flühmann: Regulatory Science, Vifor Pharma Ltd, Zürich, Switzerland
Stefan Mühlebach: Regulatory Science, Vifor Pharma Ltd, Zürich, Switzerland - Abstract:
Backgrounds
In recent years nanomedicines are becoming increasingly available. Up to 23 nanomedicines are approved, and approximately 50 are in clinical development. First followon medicinal products (nano similars) have entered the European market by applying the generic approval pathway, neglecting their nano colloidal properties. After
.. The access to the whole abstract and if available the presentation file is available to FIP members and to congress participants of that specific congress.
Please login
Last update 28 September 2023